3D Bioprinting: Gaining Traction
William Whitford, Strategic Solutions Leader, BioProcess, GE Healthcare Life Sciences
 William Whitford, Strategic Solutions Leader, BioProcess, GE Healthcare Life Sciences
William Whitford, Strategic Solutions Leader, BioProcess, GE Healthcare Life SciencesQ: Tell us a bit about yourself, and what got you into the area of bioprinting
I’m currently a Strategic Solutions Leader at GE Healthcare. I joined the company as an R&D Leader developing products supporting protein biological and vaccine production in mammalian and invertebrate cell lines. Lately, I explore emerging technologies and identify areas in which GE can contribute to the field. I also enjoy such activities as serving on the editorial advisory board for BioProcess International.
Q: What specifically is 3D bioprinting, and what special technologies does it demand?
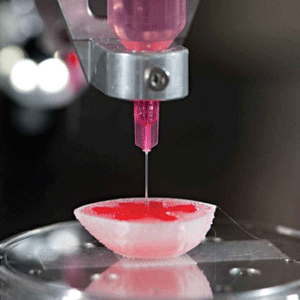
As most are aware, 3D printing (or additive manufacturing) is the creation three-dimensional products by the computer controlled deposition, or solidification, of raw materials–such as by the fusion of powder grains. An intermediate technology is the 3D printing of many diverse constructs in medicine. This include 3D printed tablets or pills, as well as the 3D printing of such biomaterials as calcium phosphates or biocompatible polymer hydrogels to be used as scaffolds in implantable biomedical devices. 3D bioprinting is quite similar, except that the final printed product contains living cells. 3D bioprinting is a deposition of micro-channels or micro-droplets of a fluid containing cells, usually with added polymeric scaffold or matrix components. This results in a firm 3D array of living cells. Often, a layer-by-layer method of depositing the “bioink” (the cell-laden solutions feeding the printers) creates 3D tissue-like structures. Many types of bioprinters are used in bioprinting, and employ such different technologies as laser-, extrusion-, and inkjet-based printing. Each technology has its own capabilities and limitations, and each requires appropriate methods and bioinks. Bioprinting exists as 2D bioprinting (laying down a monolayer of living cells with a bioprinter), 3D bioprinting (erecting3D cell-containing structures), and 4D bioprinting.
4D bioprinting is the creation of 2D or 3D objects specifically engineered to respond in predicted, anticipated ways to user-demands or a changing environment.
Like digital manufacturing in the parts and equipment world, digital biomanufacturing improves the manufacturing of biological products by using the newest computer-aided design and manufacturing technologies
The response is a self-actuated shape-morphing, cellular differentiation, tissue patterning, biological characteristic alteration or functionality development.
Q: You’ve mentioned digital biomanufacturing. What will this provide for bioprinting?
Like digital manufacturing in the parts and equipment world, digital biomanufacturing improves the manufacturing of biological products by using the newest computer-aided design and manufacturing technologies. Digital approaches here include such advances as cloud-based applications, machine learning, artificial intelligence and autonomation. In 3D bioprinting some are now generating digital (in silico) models to simulate and analyze the printer’s activity and input materials, with the goal of guiding and optimizing the printing process.
 Instruments monitoring and controlling these activates are becoming interconnected, with more and higher quality data being collected. Monitoring is improving, analysis is becoming richer, and models are beginning to describe a true predictive control-based process network”.
Instruments monitoring and controlling these activates are becoming interconnected, with more and higher quality data being collected. Monitoring is improving, analysis is becoming richer, and models are beginning to describe a true predictive control-based process network”.
Q: What are some of bioprinting’s specific products, applications, and markets?
Well, that’s a big and exciting question and can only be outlined here. 3D bioprinting is supporting basic research in such applications as creating cell, tissue and organ models of both nature and function. It is being applied in medical research in many types of advanced cell-based assays for clinical diagnostics and models of a tissue’s response to therapy.
3D printed structures have been reported to provide the best biomimetic modeling of natural tissues for viral progression assays. They’re being applied in clinical testing, assays and diagnostics–including companion diagnostics. In regenerative medicine, we are beginning to create synthetic tissues and organs for transplantation. Structures already demonstrating great success include bioprinted heart valves, cartilage, cartilaginous tissues, trachea, skin and blood vessels.
Q: What is the status of the commercialization of equipment and materials for bioprinting?
There have been CMOs providing bioprinting services for some time, as well as the commercial availability of small, inexpensive printers for research—some of which even have options providing capability for small manufacturing processes. There are a few vendors larger 3D bioprinters capable of large-scale, high-through put and regulated manufacturing. Each efficiently provides selected printing styles and production capabilities. As for materials, we are seeing more commercially available bioinks and bioink-qualified components. Beyond nutrient and shear-protection components, bioink contains high-molecular weight polymers (either natural or synthetic) that mimic an extracellular matrix to support the adhesion, proliferation, and differentiation of the component cells.
Q: What do you see in the future for bioprinting and in the applications you’ve identified?
Each bioprinting technology has advantages and limitations. Some are very accurate and precise, others are fast and move much volume. Construct design is moving from rather simple to complex, and is now incorporating many substructures and cell-types. Therefore, bioprinting is moving from simple operations to model-controlled, multi-material, interpenetrating networks using multi-mode deposition techniques. Advances are rapidly occurring from the topology of the printed structures, to the types of material components employed, to the digital techniques applied in direct the printing. So, in the printing technology itself we envision that one day application of a cloud-based platform, such as GE’s Predix™, will enable powerful manipulation of data to better support the advancing complexity of bioprinting processes. As for products, there is no doubt that bioprinting will provide new multiwall assay formats of highly organized 2D structures, and elegant biomimetic 3D models of normal and diseased tissues. We are already seeing the design of bioartificial tissue replacement therapies fabricated from cell-seeded 3D printed bioartificial nano composites, as well as vascularized tissues developed from 4D bioprinting-based constructs.
Read Also